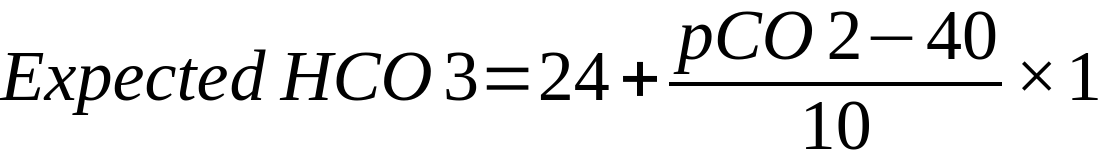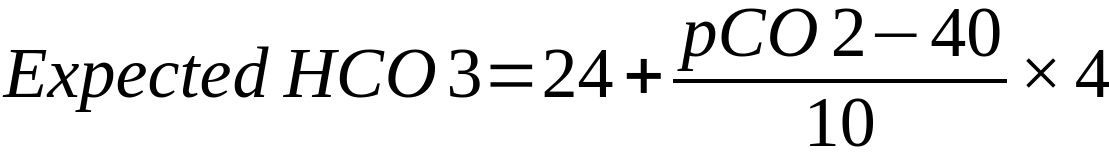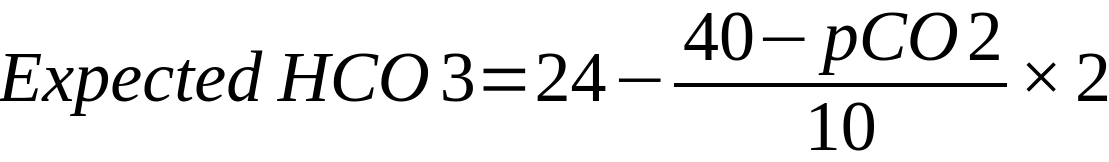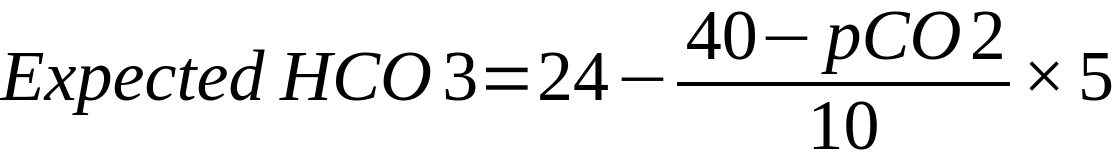By Dr Garzarella
| Metabolic acidosis
Expected pCO2 = 1.5 × [HCO3] +8 ±2 |
Metabolic alkalosis
Expected pCO2 = 0.7 × [HCO3] +20 ±5 |
| Mechanism
In metabolic acid-base disturbances, chemoreceptors sense an abnormal pH and stimulate the respiratory centre to increase or decrease ventilation to compensate Example 1. 62F presents to the emergency department with peritonitis secondary to perforated diverticulitis. She is hypotensive, tachycardic, peripherally cool and appears distressed due to pain. An ABG is taken showing: pH 7.20 pO2 95mmHg pCO2 30mmHg HCO3 18mmolL/L Na 140 mmol/L Cl 100 mmol/L Discussion Acidaemia is present Low bicarbonate and low carbon dioxide, consistent with metabolic acidosis Anion gap = 22mmol/L (high) Carbon dioxide is lower than expected Expected pCO2 = 1.5 × l[HCO3] +8 ±2 = (1.5 × 15)+8 ±2 = 33 to 37 Conclusion Metabolic acidosis (likely lactic acidosis) CO2 is lower than expected indicating a superimposed respiratory alkalosis (likely hyperventilation due to pain) |
|
| Respiratory acidosis
Acute
Chronic
|
Respiratory alkalosis
Acute
Chronic
|
| In the above formulae
24 = mid-range value of HCO3 40 = mid-range value of PaCO2 Mechanism Compensation in acute respiratory acidosis/alkalosis is limited to buffering as there is not enough time for the kidneys to respond
Compensation in chronic respiratory acidosis/alkalosis involves renal retention/loss of bicarbonate Remember ‘1 4, 2 5’: Respiratory acidosis – every 10mmHg rise in CO2 should increase HCO3– by 1 (acute) or 4 (chronic) Respiratory alkalosis – every 10mmHg decrease in CO2 should decrease HCO3– by 2 (acute) or 5 (chronic) |
|
| Example 1
57M post motor vehicle accident sustaining multiple rib fractures and a small pneumothorax. Remains hemodynamically stable. No significant past medical history. An ABG is taken showing: pH 7.23 pO2 94mmHg pCO2 58mmHg HCO3 26mmol/L Discussion Acidaemia is present High carbon dioxide, and a new injury in an otherwise healthy patient suggests an acute respiratory acidosis Bicarbonate has compensated as expected
= 25.8 Conclusion: Acute respiratory acidosis (likely due to hypoventilation secondary to pain) with appropriate metabolic compensation Example 2: 29F G1P0 at 36 weeks gestation, presents with a 2 day history of significant vomiting. pH 7.46 pO2 98mmHg pCO2 31mmHg HCO3 22mmol Discussion Mild alkalaemia is present Low carbon dioxide suggests a respiratory alkalosis, consistent with the state of pregnancy and has occurred over the space of many months Bicarbonate is higher than expected
= 19.5 Conclusion Chronic respiratory alkalosis (likely due to pregnancy) with superimposed metabolic alkalosis (likely due to vomiting) |
|
References
- Acid base disorders, Life in the Fast Lane Available at: https://litfl.com/acid-base-disorders/
- Sood P, Paul G, Puri S. Interpretation of arterial blood gas. Indian J Crit Care Med. 2010 Apr;14(2):57-64. doi: 10.4103/0972-5229.68215. PMID: 20859488; PMCID: PMC2936733.