By Dr Angus Hayes
About the author
Dr Angus Hayes is an Australian ICU registrar who recently spent time in Norway observing anaesthetic protocols and practices. In this article he examines recently published data, to compare Australian anaesthetic norms against those seen in Norway.
I walk into the OT…*BEEP* *BEEP* *BEEP*. The scents and sounds are familiar (although the language certainly proves foreign and challenging). The patient is supine. The anaesthetic machine whirs and beeps. The hustle and bustle of nurses and surgeons hurrying around the theatre makes me feel at home. One element though, is missing. The anaesthetist. I approach the anaesthetic nurse – helpfully identified by an ever-present rainbow lanyard detailing the major drugs used for paralysis, hypnosis, and analgesia. “Hvor er anestesilegen?”. I ask the nurse where the anaesthetist is. The nurse hears my bumbling, broken Norwegian and replies in English, “Oh there’s no anaesthetist for this case. It’s elective and the patient is low-risk so I run the case myself. If anything goes wrong I just hit the emergency buzzer, and the anaesthetist overseeing our group of theatres will come and assist”. My brain is spinning. Anaesthesia without an anaesthetist? This can’t be. Never during my time as an anaesthetic intern or medical student back home in Australia had I ever seen anything like this. How can a patient be put to sleep, paralysed, and operated on, without a specialist anaesthetist overseeing their care? I quickly learn that this is indeed the norm in Norway (and for other parts of Europe, apparently). Multiple cases run by multiple anaesthetic nurses with just a single anaesthetic consultant on-call for any deviations from the norm or emergencies. This was certainly unusual and foreign to me, but one could easily envision the financial benefit of this system, and it did seem to work well…or did it?
Does an absent anaesthetist make for a compromised anaesthetic? Fortuitously, a recent JAMA article aimed to answer exactly this question. “Association of Anesthesiologist Staffing Ratio With Surgical Patient Morbidity and Mortality” (Burns et al., 2022) is a retrospective, matched cohort study consisting of major noncardiac inpatient surgical procedures performed from January 1, 2010, to October 31, 2017 that was conducted in 23 US hospitals. A total of 866,453 adult patients were included. Patients were grouped by anaesthetist to patient ratios. Group 1 had a 1:1 ratio, group 2 had a 1:2 ratio, group 3 had a 1:3 ratio, group 4 had a 1:4 ratio. The measured outcomes were: 30-day mortality, and any one of 6 major surgical morbidities (cardiac, respiratory, gastrointestinal, urinary, bleeding, or infectious complications). Burns and their team subsequently found that increasing anaesthetist coverage responsibilities was associated with an increase in risk-adjusted surgical patient morbidity and mortality. Groups were batched, and when comparing groups 1-2 against groups 3-4, an increase in risk-adjusted mortality and morbidity of 14% was seen (95%CI; P<.001). Note that we see a higher mortality in the 1:1 staffing ratio group when compared to the 1:1-2 and 1:2-3 ratio groups. Burns et al postulate that this is likely due to either: 1:1 ratio cases having higher acuity and therefore higher inherent risk of mortality, OR low institutional case-loads leading to idle clinicians and lack of appropriate attention.
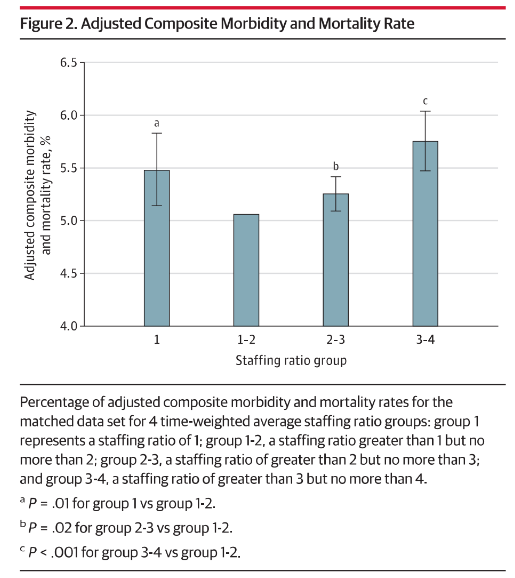
Nonetheless, we see above a clear correlation between increasing anaesthetist coverage, and increased risk of patient morbidity and mortality during non-cardiac inpatient surgical procedures. But what can we expect to gain from a system set up in this way? Financial savings and reduction in clinician workload are both important considerations that we must keep front of mind when trying to justify a system as described above. Burns et al postulate that there is a likely significant reduction in financial costs, and in clinician workload and subsequent fatigued-induced errors when such a system is utilised. Further data investigating the quantitative outcomes here are lacking and represent an area for further research.
To surmise, this author suggests that despite the increase in mortality and morbidity seen when anaesthetists are not present in the operating theatre, there are tangible financial benefits which may outweigh this burden. Further research could aim to compare associations between hospital-wide mortality and the varying levels of financial burden induced by high and low anaesthetist staffing levels at these respective institutions.