By Zheng Cheng Zhu
Key reference:
Devereaux et al. (2022). Tranexamic Acid in Patients Undergoing Noncardiac Surgery. The New England journal of medicine, 386(21), 1986–1997. https://doi.org/10.1056/NEJMoa2201171
Quick Summary
-
Tranexamic acid (TXA) is an antifibrinolytic that reversibly antagonises plasminogen’s binding site for fibrin and tissue plasminogen activator, thus preventing plasminogen activation and fibrinolysis.
-
TXA has been demonstrated to be safe and effective in reducing bleeding and improving patient outcomes in multiple critical resuscitative and operative settings, including post-partum haemorrhage, major trauma, cardiac surgery, major lower limb arthroplasties and other bleeding presentations commonly seen in the Emergency Department.
-
The Perioperative Ischemic Evaluation 3 (POISE-3) trial was a large, international multicentre placebo-controlled trial aimed to determine if perioperative TXA was safe and effective in at-risk patients undergoing non-cardiac surgeries.
-
POISE-3 found TXA considerably reduced bleeding events compared to placebo (9.1% vs 11.1%, HR 0.76, 95% CI 0.67 to 0.87; absolute difference, −2.6 percentage points; 95% CI, −3.8 to −1.4; two-sided P<0.001).
-
POISE-3 also found that TXA was associated with a small increase in cardiovascular thrombotic complications (14.2% vs. 13.9%, HR 1.03; 95% CI, 0.92 to 1.14; upper boundary of the one-sided 97.5% CI, 1.14; absolute difference, 0.3 percentage points; 95% CI, −1.1 to 1.7; one-sided P=0.04 for noninferiority), which the trial was unable to establish non-inferiority compared to placebo.
-
Nevertheless, TXA’s routine use in non-cardiac surgery with selective considerations in elevated-risk patients should be strongly encouraged given the significant improvement in bleeding-associated morbidity and mortality.
Preamble
You are an anaesthetic trainee allocated to the elective orthopaedic list. You review Mr. TX, a 56 yo M, BMI 35, arriving today for his right hip replacement for osteoarthritis. His PMHx includes:
-
Ischaemic heart disease (IHD) with 2x stents and low-normal left ventricular ejection fraction (LVEF),
-
Non-valvular atrial fibrillation (AF) and
-
Iron-deficiency anaemia (IDA) with background peptic ulcer disease (PUD). His latest Hb was 110 three weeks ago.
His medications are apixaban 5mg BD, aspirin 100mg mane, metoprolol 50mg BD, perindopril 4mg mane, paracetamol 1g QID.
Having just read the outcomes of the PREVENTT trial, you have worked-up Mr. TX pre-operatively per the Blood Management Plan for Mr.TX’s IDA:
-
Treat underlying cause of anaemia
-
Mr. TX has never had issues with iron intake
-
Mr. TX denies recent haematemesis/haematochezia or melaena, with a recent routine gastroscopy and colonoscopy showing no bleeding lesions
-
Mr. TX received an IV iron infusion per the “International Consensus Statement on the Peri-operative Management of Anaemia and Iron Deficiency”
-
-
Minimise blood loss
-
Mr. TX’s apixaban was ceased 3 days prior to day of surgery
-
Per discussion with Mr. TX’s cardiologist and your orthopaedic colleague, the team was happy for Mr. TX to continue his aspirin
-
-
Improve patient tolerance to anaemia
-
Mr. TX is ASA grade III
-
He has robust exercise tolerance with regular MET>4 activities, remained asymptomatic from his IHD or AF over last year with no hospitalisations, with an echocardiogram 3 months ago showing normal LVEF and valvular function.
-
Mr. TX has made considerable gains in lifestyle modifications, having quit smoking 3yr ago, intentionally lost weight through exercise, and has cut down on EtOH. He is keen to get his hip replaced so that he can get back on his gym routine.
-
You ask your consultant if there are any other perioperative strategies to minimise blood loss for Mr. TX, which your boss answers:
“Let’s give some tranexamic acid (TXA) before the cut”
As you nod to this conceptually-sound suggestion, you question if there is evidence that TXA reduces perioperative major bleeding, and if the theoretical increase in thromboembolic risk is significant given Mr. TX’s cardiac history…
What is TXA and its effect?
Pharmacodynamics:
Fibrinolysis, the process of fibrin clot dissolution, requires the conversion of hepatic-synthesised pro-enzyme plasminogen to its active form, plasmin, to cleave fibrin into fibrin degradation products. This activation process only occurs through direct binding of plasminogen to fibrin and interaction with tissue plasminogen activator (tPA).
TXA is a synthetic competitive reversible antagonist at the plasminogen lysine binding site for fibrin, which prevents the binding of plasminogen to fibrin and formation of plasminogen-fibrin-tPA complex. This ultimately inhibits fibrinolysis, promotes clot stability, and achieves therapeutic haemostasis. (Fig 1)
Interestingly, TXA demonstrates pleomorphic immunomodulatory effects by virtue of inhibiting plasmin-mediated activation of complement and its downstream pro-inflammatory pathways.
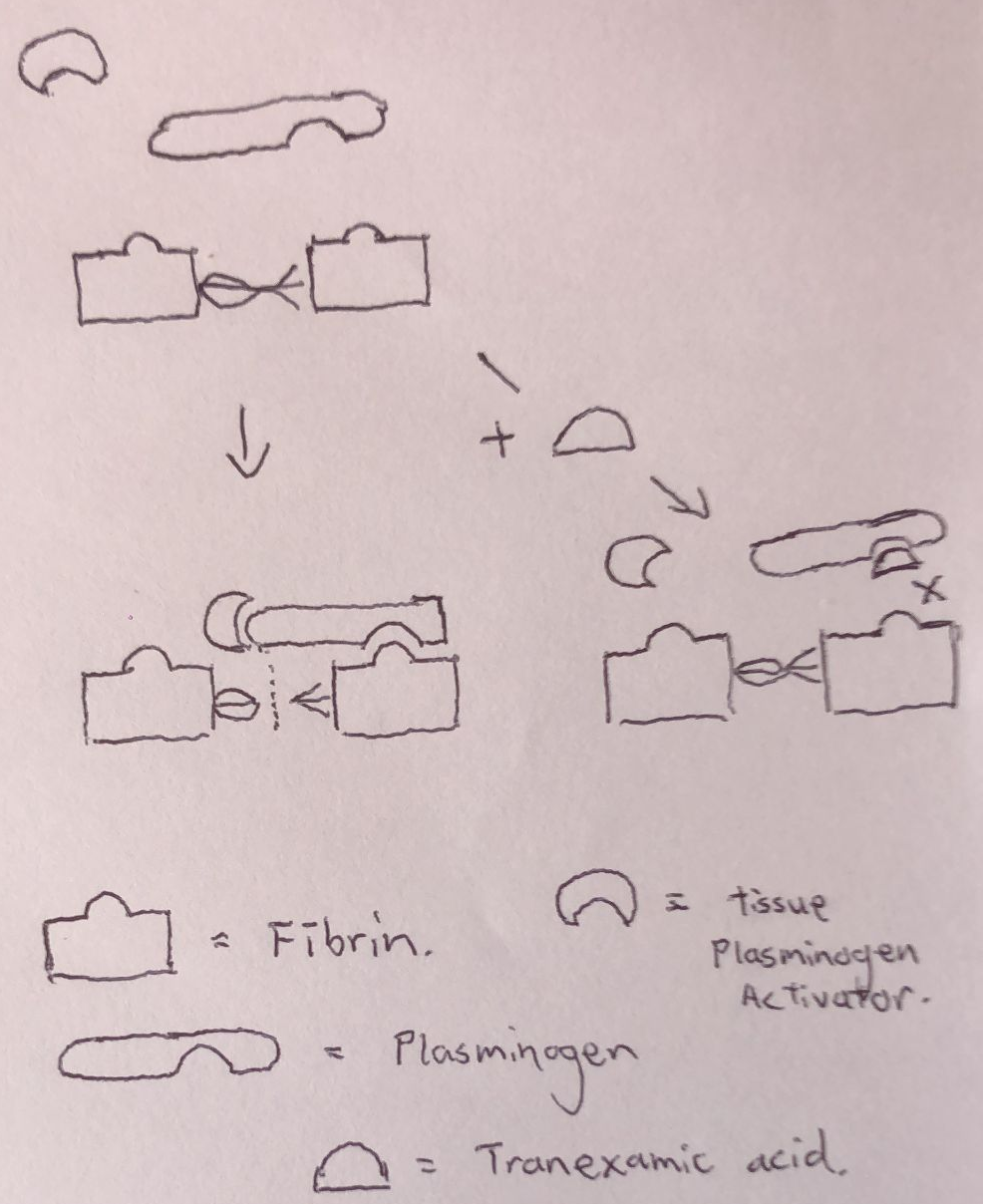
Fig 1. Simple schematic of TXA mechanism of action, depicting plasminogen competitively antagonising the plasminogen lysine receptor site, preventing plasminogen-fibrin binding and fibrinolysis.
Pharmacokinetics
Absorption:
-
Comes in IV vials 500mg in 5mls (1g/10mls), and 500mg PO tablets
-
PO bioavailability ~34%
Distribution:
-
3% protein binding with no albumin binding at therapeutic levels
-
Mostly bound to plasminogen/plasmin
Metabolism:
-
Minimal hepatic metabolism
-
Nil dosage adjustment in hepatic impairment
Elimination:
-
95% renally excreted
-
Renal half-life 2hrs, >90% cleared within 24hrs
-
Dosing consideration in renal impairment
Current indications and evidence for TXA
Post-partum haemorrhage (PPH):
PPH is the leading cause of maternal mortality worldwide, with disproportionately higher rates in low-resource countries where women have limited access to adequate obstetric care. TXA presents as an affordable intervention that can be readily administered to reduce bleeding and mortality rates.
The World Maternal Antifibrinolytic Trial (WOMAN) was an international, double-blinded placebo controlled randomised control trial (RCT) that demonstrated a significant reduction in bleeding-related mortality in women who received IV 1g TXA for PPH compared to placebo (1.5% vs 1.9%, risk ratio 0.81, 95%CI 0.65-1.00, p=0.045), with effect most pronounced when given within 3 hours from birth (RR 0·69, 95% CI 0·52-0·91; p=0·008). This benefit was not offset by increased thromboembolic events.
In light of this trial, the World Health Organisation has endorsed IV TXA given within 3 hours of birth as a key recommendation for management of PPH.

Trauma
Trauma resuscitation has evolved significantly over recent years, with increasing recognition of trauma-induced coagulopathy as a key pathology contributing to exsanguination and mortality.
It is characterised by hyperfibrinolysis and clotting factor/platelet dysfunction secondary to tissue injury, often accentuated by acidosis and hypothermia as part of traumatic haemorrhagic shock to form the “lethal triad”. As such, TXA lends itself naturally as a direct antifibrinolytic to improve haemostasis, in addition to haemorrhage control and shock reversal with blood product replacement as key priorities.
CRASH-2 was a large, international, multicentre, placebo-controlled double blinded trial investigating the effects of early administration of TXA 1g (within 3 hours of injury) on mortality, transfusion requirements and thrombotic complications. It noted:
-
Reduction in all-cause mortality (14.5% vs 16.0%, RR 0.91; 95%CI 0.85 to 0.97; p = 0.0035) and
-
Reduction in mortality associated with haemorrhage (4.9% vs 5.7%, RR 0.85; 95% CI 0.76 to 0.96; p = 0.0077), most notably in patients who received TXA within 1 hour of injury (5.3% vs 7.7%, RR 0.68; 95% CI 0.57 to 0.82; p < 0.0001)
-
no reduction in blood product requirements (50.4% vs 51.3%, RR 0.98; 95%CI 0.96 to 1.01, p=0.21
-
no increase in vascular occlusive events (1.7% vs 2.0%, 95%CI 0.68 to 1.02, p = 0.084)
-
no increase in rates of surgical management (47.9% vs 48.0%, 95%CL 0.97 to 1.02, p=0.79)
The subsequent CRASH-3 trial also demonstrated a statistically non-significant trend towards mortality benefit of early TXA for traumatic brain injury patients.
As such, early TXA (given as 1g over 10min with subsequent 1g given over 8hrs, commenced within 3hrs of injury) is now widely adopted as part of major trauma resuscitation in Australia and New Zealand.

Cardiac surgery
Cardiac surgeries carry increased risks of intraoperative bleeding, transfusion requirements, morbidity and mortality associated with poorer cardiopulmonary reserve, and need for urgent re-operation from life-threatening postoperative bleeding.
The ATACAS trial lead by Prof. Myles and colleagues aimed to investigate whether intraoperative TXA in at-risk patients undergoing coronary-artery surgery improved outcomes. It found
-
No increase in composite rates of death and thrombotic complications (nonfatal MI stroke, PE, renal failure, or bowel infarction) (16.7% vs 18.1%; RR 0.92; 95% confidence interval, 0.81 to 1.05; P=0.22)
-
Significant reduction in transfusion requirements (4331u vs 7994u, P<0.001) and rates of major haemorrhage + cardiac tamponade (1.4% vs 2.8%, P=0.001)
-
Increase in rates of seizures (0.7% vs. 0.1%, P=0.002)
Besides an increased rate of seizures, TXA is superior in preventing major bleeding complications without increase in thromboembolic events. TXA is now selectively used in our cardiac theatres for at-risk patients.
Orthopaedic surgery
In Mr. TX’s case, the use of TXA perioperatively to reduce intraoperative bleeding during elective lower limb arthroplasties is now common practice. A survey of active ANZCA fellows by Painter and colleagues in 2019 showed that 67% of our supervisors would routinely give TXA, with another 31% giving it on surgeon’s request or selectively on a case by case basis.
Not surprisingly, meta-analysis of existing small trials by Fillingham and colleagues (2018) has demonstrated that TXA, in oral, topical, or IV formulations, safely reduces risk of bleeding and need for transfusions.
It is a practice guideline in Western Health in Victoria for TXA to be administered for “all patients undergoing THR and TKR where the specialist anaesthetist providing perioperative care deems the benefits outweigh the risks, in consultation with a haematologist”.
Hereditary angioedema
Given its pleomorphic inhibition of plasmin-mediated complement activation, TXA has been used as prophylaxis as well as emergency management of hereditary angioedema with relative success in published small studies and case reports.
Emergency Department use: topical haemostatic & menorrhagia
One of the most common indications for TXA encountered in ED is menorrhagia. It reduces menstrual bleeding by 30-60%, improves women’s quality of life, and is the superior medical management compared to NSAIDS and oral contraceptives.
TXA is also used topically in ear, nose and throat & dental presentations to achieve haemostasis. Classically, TXA-soaked gauze has been used off-label for management of epistaxis. Impromptu use of 5% TXA solution (500mg tablet dissolved in 10ml) is also used for oromucosal bleeding, in particular in patients with coagulopathies.

The POISE-3 trial. Is TXA effective and safe in non-cardiac surgery?
While large trials such as ATACAS has underlined TXA’s safety and effectiveness in cardiac surgery, no large pragmatic trials exist for use of TXA in non-cardiac surgeries.
The Perioperative Ischemic Evaluation 3 (POISE-3) trial was a large, international multicentre placebo-controlled trial designed to answer whether*:
In patients at risk of bleeding and cardiovascular complications undergoing non-cardiac surgery, is perioperative TXA effective in reducing significant bleeding and safe in terms of major cardiovascular complications compared to placebo.
*The POISE-3 trial utilised a partial factorial design to also investigate the effects of hypertension avoidance vs hypotension avoidance on bleeding and cardiovascular outcomes. This was not reported in the current paper.
Study design
Patient population
-
114 hospitals across 22 countries
-
≥45yo, undergoing inpatient non-cardiac surgery (excluding intracranial neurosurgery)
-
Risk factors for bleeding and cardiovascular complications
-
Age ≥70
-
Major surgery
-
History of atherosclerotic / coronary artery disease
-
History of malignancy
-
History of chronic kidney disease (creatinine > 175micromol/L)
-
Randomisation to Treatment and control
-
1:1 randomisation
-
“TXA” group = 1g TXA bolus at start and end of case
-
“Control” group = Placebo at start and end of case
Outcomes
-
Primary Efficacy outcome: composite of life-threatening bleeding, major bleeding, bleeding into critical organ at 30 days
-
Primary Safety outcome: composite of myocardial injury, ischaemic stroke, peripheral arterial thrombosis, proximal VTE at 30 days
-
Secondary outcomes included:
-
Individual components of composite efficacy and safety outcomes
-
Bleeding independently associated with death
-
Patient requiring transfusions
-
LOS and days alive at home
-
Seizures
-
Statistical design
-
To satisfy efficacy hypothesis, that TXA is superior to placebo = two side P<0.05
-
To satisfy safety hypothesis, that TXA is non-inferior to placebo = upper end of one-sided 97.5% CI must <1.125, and one-sided P<0.025, corresponding to risk increase of <12.5%
-
9500 recruited to achieve 90% power to detect hazard ratio (HR) of 0.8 or less for efficacy hypothesis (assuming 9% bleeding event at baseline), and 98% power for non-inferiority margin for HR <1.125 (assuming 14% cardiovascular event at baseline)
-
Efficacy outcome analysed in intention-to-treat (ITT) population
-
Safety outcome analysed in per-protocol (PP) and ITT population per sensitivity analysis
-
Cox proportional-hazards models were used to calculate HR
Results
Total 9537 patients were randomised to TXA n=4757 and placebo n=4778, with similar demographic baselines
Primary outcomes
-
Efficacy outcome
-
TXA was superior compared to placebo in preventing major bleeding events by 24% (9.1% vs 11.1%, HR 0.76, 95% CI 0.67 to 0.87; absolute difference, −2.6 percentage points; 95% CI, −3.8 to −1.4; two-sided P<0.001)
-
-
Safety outcome
-
TXA could not be deemed non-inferior compared to placebo in cardiovascular outcomes
-
PP population (14.2% vs. 13.9%, HR 1.03; 95% CI, 0.92 to 1.14; upper boundary of the one-sided 97.5% CI, 1.14; absolute difference, 0.3 percentage points; 95% CI, −1.1 to 1.7; one-sided P=0.04 for noninferiority)
-
ITT sensitivity analysis HR 1.03; 95% CI, 0.92 to 1.14
-
-
Secondary outcomes
-
TXA superior in preventing
-
Bleeding independently associated with death by 24%
-
8.7% vs. 11.3%, HR 0.76, 95%CI 0.67 to 0.87
-
-
Major bleeding by 28%
-
7.6% vs. 10.4%, HR 0.72, 95%CI 0.63 to 0.83
-
-
Number of patients requiring transfusions
-
9.4% vs. 12.0%, odds ratio 0.77, 95% CI, 0.68 to 0.88
-
-
-
TXA was not associated with statistically significant increase in risk of individual cardiovascular events (assuming 95% CI and p<0.05, differing from non-inferiority requirements)
Risk-benefit implications
The POISE-3 trial has clearly demonstrated that TXA is effective in reducing major bleeding and its associated morbidity and mortality (by 2.6 absolute percentage points or relative 24% reduction) in non-cardiac surgery, and presents an attractive, cost-effective intervention to improve clinical outcomes and reduce costs in terms of blood transfusions. Nevertheless, clinicians must also balance the theoretical 3% increase in cardiovascular complications, particularly in those at increased risk (recent VTE events, strokes, or active coronary artery disease). Ultimately, taking into account the significant gains in bleeding outcomes compared to the small probability of complications, one may argue for its routine use in non-cardiac surgery with selective considerations in elevated-risk patients.
References
CRASH-2 trial collaborators, Shakur, H., Roberts, I., Bautista, R., Caballero, J., Coats, T., Dewan, Y., El-Sayed, H., Gogichaishvili, T., Gupta, S., Herrera, J., Hunt, B., Iribhogbe, P., Izurieta, M., Khamis, H., Komolafe, E., Marrero, M. A., Mejía-Mantilla, J., Miranda, J., Morales, C., … Yutthakasemsunt, S. (2010). Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet (London, England), 376(9734), 23–32. https://doi.org/10.1016/S0140-6736(10)60835-5
CRASH-3 trial collaborators (2019). Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet (London, England), 394(10210), 1713–1723. https://doi.org/10.1016/S0140-6736(19)32233-0
Chauncey JM, Wieters JS. Tranexamic Acid. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532909/
Fillingham, Y. A., Ramkumar, D. B., Jevsevar, D. S., Yates, A. J., Shores, P., Mullen, K., Bini, S. A., Clarke, H. D., Schemitsch, E., Johnson, R. L., Memtsoudis, S. G., Sayeed, S. A., Sah, A. P., & Della Valle, C. J. (2018). The Efficacy of Tranexamic Acid in Total Hip Arthroplasty: A Network Meta-analysis. The Journal of arthroplasty, 33(10), 3083–3089.e4.
Leminen, H., & Hurskainen, R. (2012). Tranexamic acid for the treatment of heavy menstrual bleeding: efficacy and safety. International journal of women’s health, 4, 413–421. https://doi.org/10.2147/IJWH.S13840
Maj Richard Reed, LtCol Tom Woolley, Uses of tranexamic acid, Continuing Education in Anaesthesia Critical Care & Pain, Volume 15, Issue 1, February 2015, Pages 32–37, https://doi.org/10.1093/bjaceaccp/mku009
Myles, P. S., Smith, J. A., Forbes, A., Silbert, B., Jayarajah, M., Painter, T., Cooper, D. J., Marasco, S., McNeil, J., Bussières, J. S., McGuinness, S., Byrne, K., Chan, M. T., Landoni, G., Wallace, S., & ATACAS Investigators of the ANZCA Clinical Trials Network (2017). Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. The New England journal of medicine, 376(2), 136–148. https://doi.org/10.1056/NEJMoa1606424
Pabinger, I., Fries, D., Schöchl, H., Streif, W., & Toller, W. (2017). Tranexamic acid for treatment and prophylaxis of bleeding and hyperfibrinolysis. Wiener klinische Wochenschrift, 129(9-10), 303–316. https://doi.org/10.1007/s00508-017-1194-y
Painter TW, McIlroy D, Myles PS, Leslie K. A survey of anaesthetists’ use of tranexamic acid in noncardiac surgery. Anaesthesia and Intensive Care. 2019;47(1):76-84. doi:10.1177/0310057X18811977
Nickson (2020). Tranexamic acid. Life In The Fast Lane. Accessed on 25/09/23. URL: https://litfl.com/tranexamic-acid/
Schutgens, Roger E. G.1; Lisman, Ton2. Tranexamic Acid Is Not a Universal Hemostatic Agent. HemaSphere 5(8):p e625, August 2021. | DOI: 10.1097/HS9.0000000000000625
WOMAN Trial Collaborators (2017). Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet (London, England), 389(10084), 2105–2116. https://doi.org/10.1016/S0140-6736(17)30638-4