By Luke Chan
Key reference:
Velayudhan, Bellingham & Morley-Forster. (2014). Opioid-induced hyperalgesia. Continuing Education in Anaesthesia, Critical Care and Pain, Volume 14, Issue 3, 125 – 129, https://doi.org/10.1093/bjaceaccp/mkt045
Background
-
What is Opioid induced Hyperalgesia (OIH)?
-
Patients treated with opioids who paradoxically demonstrate increased sensitivity to painful stimulus
-
-
Effectiveness of high-dose opioids can be diminished by OIH and opioid tolerance – difficult to distinguish
-
Evidence primarily from observational studies of patients exposed to long-term methadone maintenance therapy for treatment of substance dependence
Clinical features
-
Hyperalgesia (increased response to painful stimuli)
-
Allodynia (painful response to normally innocuous stimulus)
-
Different region and different quality to original pain
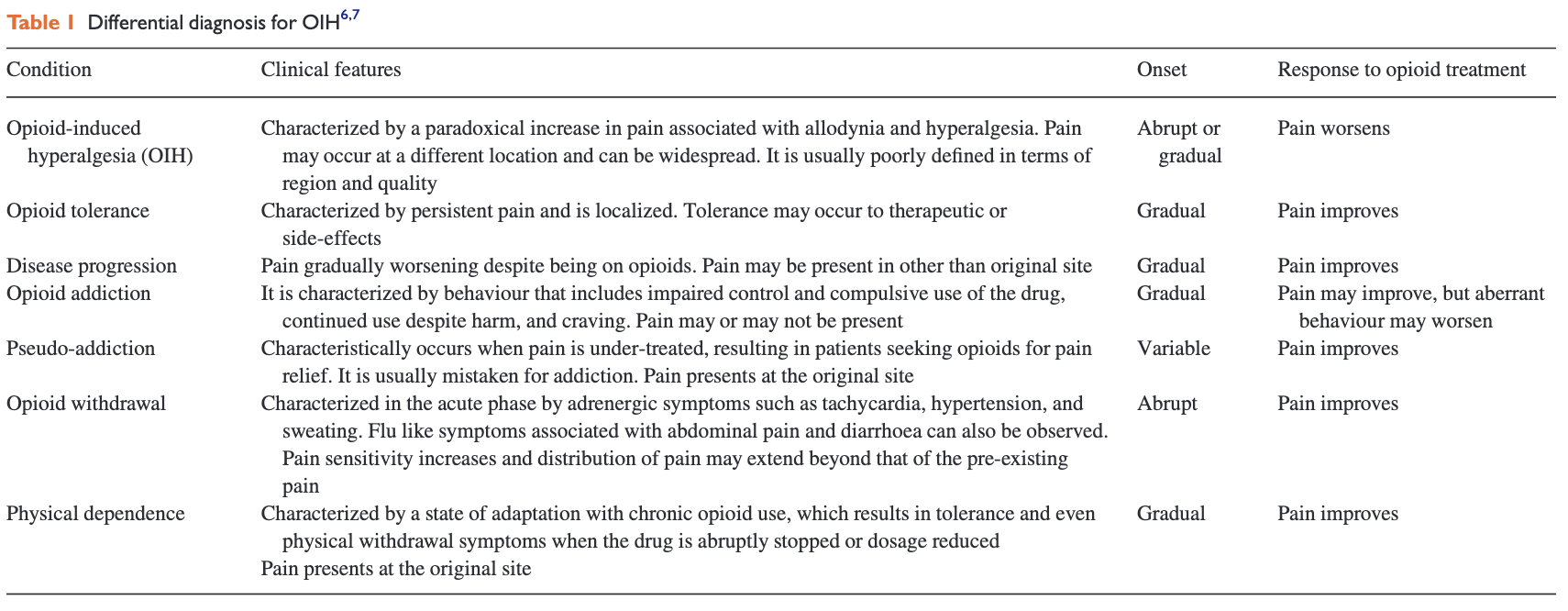
Differential Diagnoses
Figure from https://www.bjaed.org/article/S1743-1816(17)30101 4/fulltext#:~:text=Opioid%2Dinduced%20hyperalgesia%20(OIH),increased%20sensitivity%20to%20painful%20stimuli.
Opioid receptor physiology
-
4 types of receptors: MOP (µ-opioid peptide receptor), DOP (δ-opioid peptide receptor), KOP (κ-opioid peptide receptor), and NOP (nociception/orphanin FQ peptide receptor)
-
Distributed widely in brain, spinal cord, peripheral afferent nerve terminals, and other organs
-
Opioid-sensitive neurons in rostral ventral medulla may facilitate OIH
Pathophysiology
Although the exact mechanism is not clearly understood, current hypotheses include central and peripheral mechanisms
Central mechanisms
|
Central glutaminergic system |
Acute and chronic opioid use increases NMDA receptor activity Prolonged morphine administration -> down-regulation of spinal glutamate transporters in spinal cord -> increased glutamate levels available -> spinal neurone sensitisation |
|
Spinal dynorphins |
Dynorphins (opioid peptides) increases with continuous infusions of µ-receptor agonists Increased dynorphins -> release of excitatory neuropeptides Excitatory neuropeptides act as pronociceptive agents -> enhanced nociceptive inputs at spinal level |
|
Descending facilitation |
ON cells and OFF cells in rostral ventral medulla facilitate and inhibit pain signals respectively Opioid-sensitive ON cells mediate descending facilitation -> promote spinal nociceptive processing |
|
Change in opioid receptor responsiveness |
Chronic exposure to opioids -> alteration of G-protein activity (conversion from inhibitory to excitatory-coupled mode) -> increase in excitatory activity |
Peripheral mechanisms
|
Paradoxical serotonergic receptor activity |
Activation of serotonergic receptors -> shifts balance from descending inhibitory control towards pro-nociception |
|
Substance P |
Neurotransmitter released by C-fibre neurons that augments postsynaptic neuron effects of glutamate |
|
Alteration to neuron cellular environment |
Alteration of cytokine production, calcium channels and nitric oxide synthetase |
How do we diagnose OIH?
-
No specific test or exam to confirm OIH
-
Establish diagnosis by increasing dose of opioids and evaluating for increased efficacy. Also rule out progression of disease or new pathology
-
Quantitative sensory testing
-
Being investigated as diagnostic tool
-
Involves applying different mechanical and thermal stimuli to measure pain threshold
-
How can we prevent OIH?
-
Reducing total dose of opioid
-
Pharmacotherapy: combination of anticonvulsants, antidepressants, and NSAIDs
-
Interventional therapy: regional blocks, peripheral nerve blocks, and spinal cord stimulation can assist in diagnosis pain generators and provide therapeutic benefit
-
Psychological therapy: studies have shown effectiveness of cognitive behavioural therapy in chronic pain
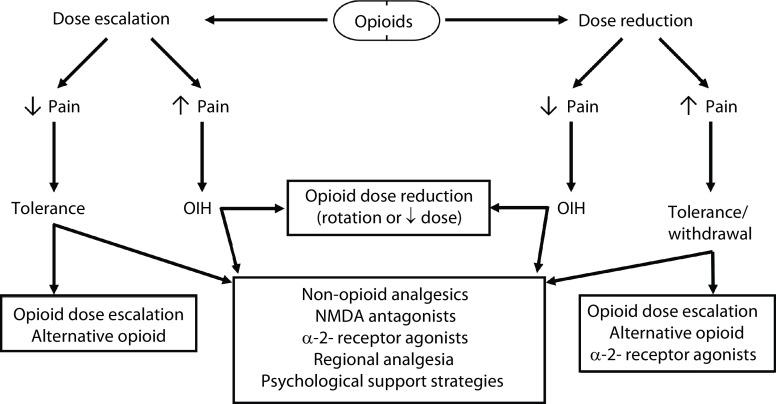
How do we manage OIH?
-
Establish diagnosis by increasing dose of opioids and evaluating for increased efficacy
-
Opioid dose reduction
-
Reduction of 40-50% and substitution with low-dose opioid agonist such as methadone
-
-
Opioid rotation/switching
-
When converting from one opioid to another, decrease dose of new opioid by 25-50% to account for incomplete cross-tolerance
-
Fentanyl, methadone and buprenorphine are commonly used in switching
-
Utilise opioid sparing adjuvants (e.g. NSAID, acetaminophen, anticonvulsant, antidepressant)
-
Methadone
-
-
Advantages: can act as an NMDA antagonist in addition to opioid agonism and norepinephrine and serotonin reuptake inhibition, incomplete cross-tolerance, relatively long half-life (24-36h)
-
Disadvantages: drug interactions are more frequent that with other long-acting opioids, complex conversions
-
Buprenorphine
-
-
Superior safety profile
-
Lower risk of abuse
-
May return to eliciting hyperalgesia over time
-
Fentanyl
-
-
Physicochemical properties make it ideal to be delivered via transdermal route
-
Low molecular weight, high lipophilicity, high potency, and optimal skin flux
-
-
Opioid-sparing agent: NMDA antagonists
Ketamine: non-competitive NMDA antagonist
-
-
Available as racemic mixture or S-ketamine isomer
-
Subcut or IV: 0.125-0.3mg/kg/h
-
Oral: start does not require intensive monitoring but must be produced by a pharmacist. Start with 0.5mg/kg racemic mixture or S-ketamine -> increase by 0.25-0.5mgkg in stepwise manner
-
3-4 times per day due to short half-life of 2.5h
-
Not comprehensively studied
-
Dextromethorphan: non-competitive NMDA antagonist
-
-
Significant clinical effectiveness yet to be demonstrated
Figure from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8023328/

-