Dr Wendell Zhang
Disclaimer
This is an educational piece and is not intended to provide medical advice. Please refer to your local guidelines for specific details regarding this topic.
Key points
-
Acquired disorders of coagulation present several challenges in the perioperative space; patients with these disorders require optimisation before their operation
-
In this patient group, it is important to have a multidisciplinary discussion with a haematologist and the surgeon to mitigate the risk of bleeding
-
Prophylactic transfusion of platelets has not been definitively shown to improve patient outcomes nor does it reduce the need for a blood transfusion in patients undergoing non-cardiac surgery with thrombocytopaenia
-
INR is not a reliable indicator for risk of bleeding in patients with liver cirrhosis
-
Prophylactic transfusion of plasma in patients with cirrhosis is not associated with better clinical outcomes
-
Disseminated intravascular coagulation (DIC) impairs many aspects of haemostasis and is associated with significant morbidity and mortality
Preamble
You are the anaesthetic registrar and have been called to perform a perioperative review. The patient is Ms Gray, a 75-year-old woman who sustained a left periprosthetic fracture following a mechanical fall. Ms Gray also has a history of atrial fibrillation and is taking warfarin daily with her INR currently being 5.2.

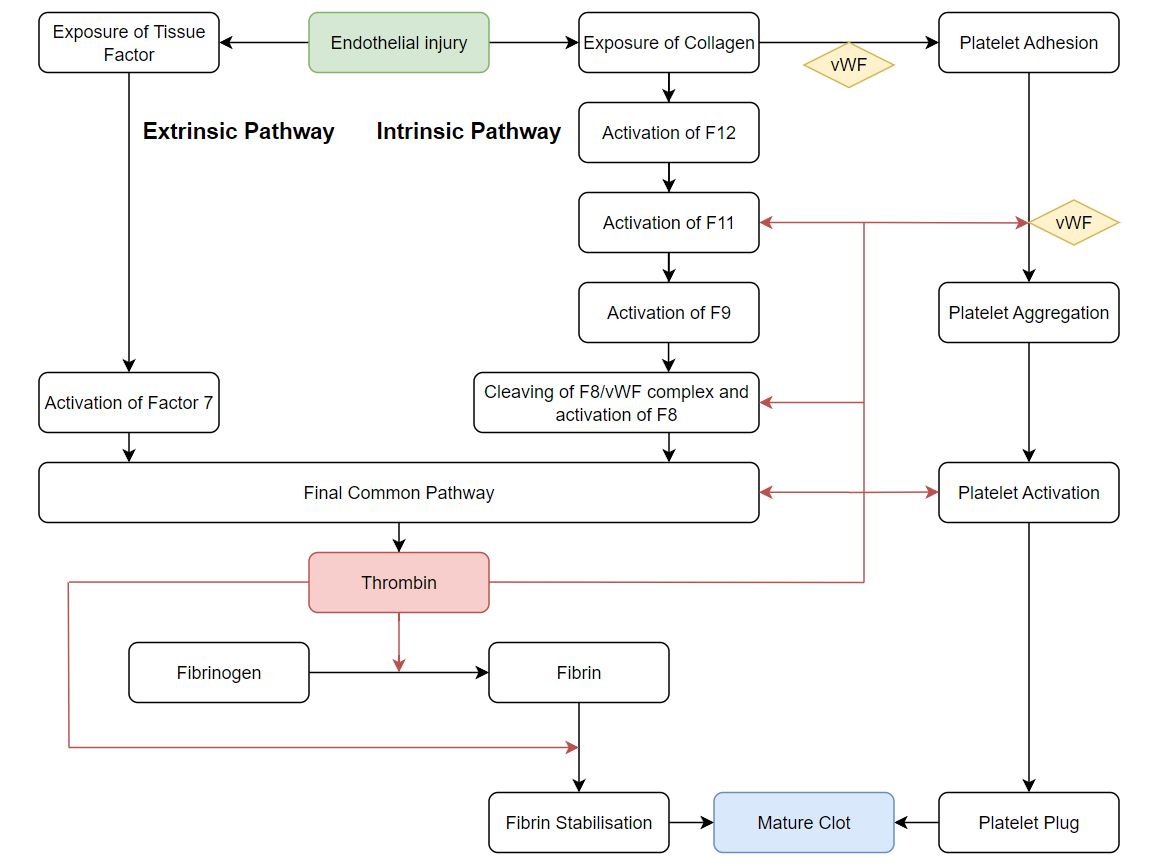
Introduction and a brief rehash of haemostasis
A common severe intra-operative and post-operative surgical complication is uncontrolled bleeding. This risk is exacerbated in patients who have a pre-existing disorder of coagulation. Anaesthetists will frequently come across such patients and will be called upon to optimise these issues preoperatively.
The broad strokes of the coagulation process can be found in this article (please provide a link to the other blog post). At a glance, the body responds to damage to its vascular endothelium by activating various intersecting pathways to achieve a mature clot to prevent further bleeding.

Haemostasis broadly consists of two simultaneous processes:
-
Primary Haemostasis: Platelet accumulation at the site of injury to form a “platelet plug”
-
Secondary Haemostasis: Reinforcing the platelet plug with a “fibrin plug”
When discussing patients with acquired disorders of haemostasis, it is likewise useful to break them down into these two categories.
Acquired Disorders of Primary Haemostasis
Primary haemostasis is the initial response to vascular injury and revolves around the early interactions between platelets and the site of damage. As such, deficiencies in this process revolve around platelets.
Platelets are fragments of cells produced by megakaryocytes in the bone marrow. The production of platelets is regulated by thrombopoietin released from the kidney and liver. Platelets circulate in the bloodstream for an average of 10 days and are held in reserve in the spleen.
Disorders in primary haemostasis can be further broken down into 3 main groups:
|
Disorder of Platelet Production |
Key Mechanism |
|
Hepatic/Renal Disease |
Decreased secretion of thrombopoietin |
|
Suppression of Bone marrow |
Secondary to malignant infiltration, viral infection, chemotherapy |
|
B12/Folate Deficiency |
Impairs key enzymes in platelet production |
|
Disorders of Excess Platelet Destruction |
Key Mechanism |
Management |
|
Immune Thrombocytopaenic Purpura |
Autoimmune destruction of platelets |
Steroids, IVIG, splenectomy |
|
Thrombotic Thrombocytopaenia |
Deficiency in ADAMTS13 resulting in the creation of microthrombi and subsequent platelet consumption |
Plasma exchange, steroids |
|
Haemolytic Uraemic Syndrome |
Shiga toxin initiates the creation of microthrombi with increased platelet consumption |
Supportive treatment, monitoring of electrolytes and renal function |
|
HELLP Syndrome |
Associated with pre-eclampsia/eclampsia with platelet consumption in vessels surrounding the placenta |
Delivery of the baby, antihypertensives, monitoring of Hb levels, platelet transfusion if bleeding |
|
Heparin Associated Thrombocytopaenia |
Heparin forms a complex with platelets which results in excess platelet activation and consumption |
Cease heparin, consider alternative anticoagulation to mitigate risk of thrombosis |
|
Severe Congestive Liver Disease |
Platelet sequestration in the spleen |
Typically does not require acute treatment |
|
Disorders of Platelet Function |
Key Mechanism |
Management |
|
Renal Failure |
Interplay between uraemia and abnormal platelet-endothelial interaction |
Desmopressin, consideration of dialysis |
|
Aspirin |
Irreversible inactivation of COX which prevents formation of thromboxane |
Withhold (patients will take ~1 week to recover full platelet function). Rarely platelet transfusion. |
|
ADP receptor inhibitors (clopidogrel, ticagrelor, prasugrel) |
Inhibition of platelet activation and aggregation |
Withhold. Rarely platelet transfusion. |
|
Glycoprotein 2b/3a receptor inhibitors (abciximab, tirofiban, eptifibatide) |
Inhibition of platelet aggregation |
Withhold. Rarely platelet transfusion. |
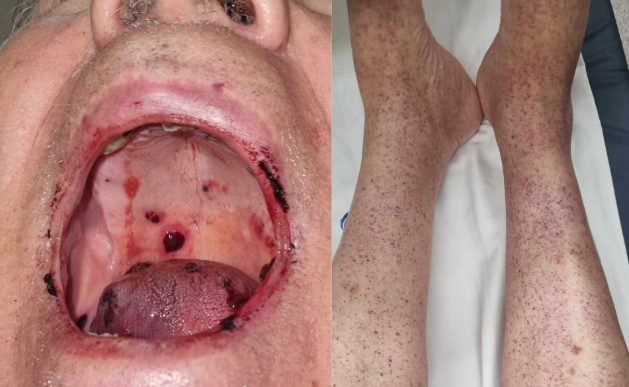
Patients with disorders of primary haemostasis may present with mucocutaneous bleeds (from gums and oral mucosa) or peripheral ecchymosis. A normal platelet count ranges between 150 – 450 x 10^9/L. In these patients, the coagulation profile (APTT/PT/INR) is typically normal.
There is no clear linear relationship between platelet levels and the risk of clinically significant bleeding. However, patients with severe thrombocytopaenia (below 10 x 10^9/L) are prone to spontaneous bleeding with there being an increased risk of clinically significant bleeding events.
Platelet Transfusions

There is a lack of strong evidence regarding perioperative optimisation of patients with deficiencies in primary haemostasis. Prophylactic transfusion of platelets to treat thrombocytopaenia has not been definitively shown to improve patient outcomes nor does it reduce the need for a blood transfusion in patients undergoing non-cardiac surgery. Platelet transfusion is indicated if there isis severe or life threatening active bleed (e.g trauma, disseminated intravascular coagulopathy (DIC), bleeding into critical sites (e.g CNS, eye), and the aetiology is suspected to be from thrombocytopenia.
Nevertheless, the National Blood Authority’s Patient Blood Management Guidelines have listed acceptable indications for prophylactic platelet transfusions for prevention of bleeding. Of note, it has recommended transfusion for patients undergoing any “invasive procedures”, which applies to most patients having major surgery.
|
Acceptable indications |
Platelet threshold (x10^9/L) |
|
Neurosurgery |
<100 |
|
Invasive procedures |
<50 |
|
Vaginal or Caesarean birth |
<50 |
|
Central venous access insertion |
<20 |
|
Critically ill patients |
<20 |
|
Pre-term / low birth weight infants |
<20 |
|
Preterm neonate with Foetal and Neonatal Alloimmune Thrombocytopenia (FNAIT) |
<50 |
Platelet transfusion is to be undertaken with care. In addition to typical adverse events associated with blood product transfusion, there is an increased risk of infection either by transmission from the product or via immunosuppression post-transfusion. If in doubt, double check your local guidelines or obtain Haematology advice.
For patients receiving a platelet transfusion, it is also important to recheck the FBE 30 minutes following the transfusion to check for an appropriate increment using either Corrected Count Increment (CCI) or Percentage Platelet Recovery (PPR) formulae.
Antiplatelet Medications
Antiplatelet medications inhibit various aspects of platelet function. Aspirin binds irreversibly to COX and prevents the formation of thromboxane which is important for platelet activation. ADP receptor inhibitors (clopidogrel, ticagrelor, prasugrel) and Glycoprotein 2b/3a receptor inhibitors (abciximab, tirofiban, eptifibatide) impair the activity of platelet surface receptors and thereby impair platelet activation and aggregation. For patients taking antiplatelet medications, there are currently no routinely used antidotes for reversal. Typically, patients are instructed to withhold these medications prior to elective procedures. In an emergency setting, the transfusion of platelets may be indicated along with the administration of desmopressin.
Additional therapeutic options include the use of desmopressin to augment platelet function by inducing the release of vWF. It has been demonstrated to be effective in patients with uraemia-driven platelet dysfunction.
Acquired Disorders of Secondary Haemostasis
Secondary Haemostasis describes the process where fibrinogen is converted into a fibrin plug to provide support to the immature clot. This process is dependent on the action of two key pathways which induce the cascading activation of clotting factors – the intrinsic and extrinsic pathways.
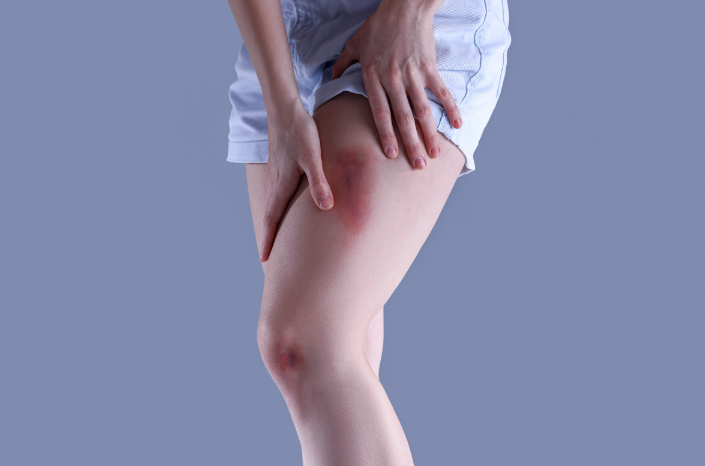
Patients with clinically significant disorders of secondary haemostasis present with excessive bleeding, bruising, haematomas and haemarthrosis. Patients may also have deranged coagulation studies (INR/APTT). To further characterise the coagulation disorder, mixing studies may be performed. In this study, the patient’s blood is combined with normal plasma which contains normal functioning clotting factors. In general, clotting disorders which are corrected with mixing studies are caused by a lack of clotting factors whereas those that persist are typically caused by clotting factor inhibition.
|
Disorders of Secondary Haemostasis |
Mechanism |
Management |
|
Nutritional Deficiency of Vitamin K |
Deficiencies in Factor 2,7,9,10 |
Vitamin K infusion, prothrombinex |
|
Warfarin |
Deficiencies in Factor 2,7,9,10 |
Withhold, Vitamin K infusion, prothrombinex, activated charcoal in overdose |
|
NOAC |
Inhibition of Factor 10a, thrombin (dabigatran) |
Withhold, idarucizumab (for dabigatran) |
|
Thrombolytic Agents |
Activation of plasmin and breakdown of fibrin |
Withhold, cryoprecipitate, tranexamic acid. |
|
Heparin |
Activation of antithrombin 3 and deactivation of thrombin and Factor 10a |
Protamine |
|
Liver Failure |
Reduced synthesis of clotting factors and anticoagulation factors |
Consideration of Vitamin K infusion, FFP, platelets |
|
Disseminated Intravascular Coagulation |
Systemic consumption of coagulation factors, fibrinogen and platelets |
Treat primary cause, consider plasma and factor concentrate infusions |
Vitamin K Deficiency and Warfarin

The clotting factors are almost all synthesised in the liver and most take the form of inactive proteins in circulation. Of note, clotting factors 2,7,9,10 are dependent on vitamin K to be produced in a biologically active form and have short half-lives. In addition, the synthesis of factors regulating coagulation such as Protein C and S are also dependent on vitamin K. Vitamin K is a fat-soluble vitamin found in leafy green vegetables. Warfarin reduces the bioavailability of active vitamin K and these factors become rapidly depleted given their short half-lives. The therapeutic effect of warfarin is measured using the patient’s PT/INR. Reversal of warfarin involves the administration of IV vitamin K (between 0.5 to 10mg). Rarely, patients may require prothrombin complex concentrate (prothrombinex) which contains Factor 2,9,10 in addition to Vitamin K for very rapid reversal. The administration of prothrombotic agents all carry an increased risk of thrombotic events and must be considered carefully. Fresh frozen plasma (FFP) is not frequently used to reverse warfarin in the acute setting but it may be used as a substitute for prothrombinex for acute reversal.
|
Key Clotting Factors |
Pathway |
Synthesis |
|
1 (Fibrinogen) |
Common |
Liver |
|
2 (Prothrombin) |
Common |
Liver – Vitamin K dependent |
|
3 (Tissue Factor) |
Extrinsic |
Damaged cells |
|
4 (Calcium ions) |
All |
Plasma |
|
5 |
Common |
Liver |
|
7 |
Extrinsic |
Liver – Vitamin K dependent |
|
8 |
Intrinsic |
Platelets |
|
9 |
Intrinsic |
Liver – Vitamin K dependent |
|
10 (Thrombokinase) |
Common |
Liver – Vitamin K dependent |
|
11 |
Intrinsic |
Liver |
|
12 |
Intrinsic |
Liver |
|
13 (Fibrin Stabilising Factor) |
None |
Liver, platelets |
Medication-mediated Disorders of Secondary Haemostasis

Novel Oral Anticoagulants (NOACs) act by inhibiting Factor 10a (rivaroxaban/apixaban) or thrombin-mediated factor activation (dabigatran). The therapeutic activity of NOACs is measured using specific clotting assays which may not be routinely available. Reversal of dabigatran may be achieved with idarucizumab however this is associated with significant cost and is not routinely available. Rapid reversal of rivaroxaban and apixaban may be achieved with prothrombinex or adexanet-alfa which is likewise not widely available. Antidotes for NOACs are relatively new medications and evidence regarding clinical efficacy is emerging.
Unfractionated heparin activates antithrombin 3 which allows for deactivation of thrombin and factor 10a. Compared to Low Molecular Weight Heparin (LMWH) such as enoxaparin, it has a short half life and is more easily reversible. Reversal of unfractionated heparin (and to lesser extent LMWH)can be achieved with protamine as it binds irreversibly to these agents. Patients with allergies to fish may be at increased risk of allergic reactions to protamine infusions.
Thrombolytic agents such as streptokinase and alteplase work by activating plasminogen into plasmin which is used to break down fibrin and fibrinogen in clots. Reversal of these agents is typically indicated for significant bleeding events and may be achieved with cryoprecipitate (contains fibrinogen) and tranexamic acid.
Liver failure
For patients with cirrhosis and impaired synthetic liver function, INR is not a reliable indicator of their risk of bleeding. In this patient group, the impaired synthetic function results in both the reduced production of clotting factors as well as key anticoagulant factors. Patients may paradoxically be both at increased risk of haemorrhage and thrombosis. Interventions such as routine prophylactic FFP transfusion do not reliably correct laboratory coagulation abnormalities and is not recommended. Likewise, routine prophylactic transfusion of cryoprecipitate is not strongly associated with better perioperative outcomes and is not recommended.
Alternative studies such as TEG (Thromboelastography) and ROTEM have been introduced to provide a clearer picture into coagulation defects and have been suggested to better reflect haemostasis in vivo. While analysis with TEG/ROTEM has been demonstrated to be effective in certain settings, they are limited in their availability.
Disseminated Intravascular Coagulation
Disseminated Intravascular Coagulation (DIC) is characterised by widespread activation of haemostatic processes mediated by abnormal exposure of tissue factor in response to a systemic inflammatory response. Causes of DIC can be broadly split into infective (sepsis) and non-infective (trauma, malignancy, acute promyelocytic leukaemia, vasculitis among various others). The systemic activation of the coagulation pathways alongside suppression of innate anticoagulation mechanisms creates innumerable microthrombi. This prothrombotic state evolves into a consumptive coagulopathy as platelets and clotting factors are exhausted systemically. The prognosis of DIC can be quite poor and evidence surrounding the management of the associated coagulopathy does not point to a definite modality of treatment. Ultimately, patients with DIC will require treatment for the precipitating event with the FFP and concentrated factor infusions for patients at risk of bleeding.
Preoperative Assessment and Optimisation

As with all preoperative assessments, a detailed history should be obtained from the patient if possible. In particular, the patient’s medication and adherence to those medications should be assessed. It would also be important to establish the patient’s nutritional status if there are concerns regarding vitamin K deficiency. On examination, care should be taken to examine for features of liver disease as well as mucosal, intramuscular, intraarticular or subcutaneous bleeding.
Pre-operative investigations should also include an FBE, UEC, LFT, coagulation studies, Group and Hold as well as specific clotting factor assays, anti-factor Xa level and TEG if warranted.
Patients with disorders of coagulation are medically quite complex, especially those requiring an acute surgical intervention. Close collaboration with surgery, haematology and pathology teams is necessary to navigate these challenging cases.
References:
Ghadimi K, Levy JH, Welsby I2. Perioperative management of the bleeding patient. BJA: British Journal of Anaesthesia. 2016 Dec 1;117(suppl_3):iii18-30.
Levy JH, Azran M. Anesthetic concerns for patients with coagulopathy. Current Opinion in Anesthesiology. 2010 Jun 1;23(3):400-5.
Lifeblood. Use of platelets. Australian Red Cross. URL https://www.lifeblood.com.au/health-professionals/clinical-practice/use-of-blood-components/use-of-platelets [Last accessed 23/01/2024]
Ghadimi K, Levy JH, Welsby I2. Perioperative management of the bleeding patient. BJA: British Journal of Anaesthesia. 2016 Dec 1;117(suppl_3):iii18-30.
Nagrebetsky A, Al-Samkari H, Davis NM, Kuter DJ, Wiener-Kronish JP. Perioperative thrombocytopenia: evidence, evaluation, and emerging therapies. British Journal of Anaesthesia. 2019 Jan 1;122(1):19-31.
Warner MA, Jia Q, Clifford L, Wilson G, Brown MJ, Hanson AC, Schroeder DR, Kor DJ. Preoperative platelet transfusions and perioperative red blood cell requirements in patients with thrombocytopenia undergoing noncardiac surgery. Transfusion. 2016 Mar;56(3):682-90.
Krishnegowda M, Rajashekaraiah V. Platelet disorders: an overview. Blood Coagulation & Fibrinolysis. 2015 Jul 1;26(5):479-91.
Harrison MF. The misunderstood coagulopathy of liver disease: a review for the acute setting. Western Journal of Emergency Medicine. 2018 Sep;19(5):863.
Yip SW, Chan YC. Antidotes for patients taking novel oral anti-coagulants. World Journal of Emergency Medicine. 2015;6(4):311.